The process of electronic delocalization of hydrocarbons
In physical chemistry, the delocalized electron is a molecule not associated with an atom or a covalent bond and which is part of an electronic system that extends to adjacent atoms. In this way, conjugated systems with double bonds and aromatic systems come to life.
The same process of electronic delocalization occurs in solid metals where the d orbital interferes with the upper s orbital. In this case, there are positive cations and ions that line up in a sea of electrons, which are free to move in the structure. This allows the metal to develop certain characteristics such as electrical conductivity.
The electronic delocalization of benzene
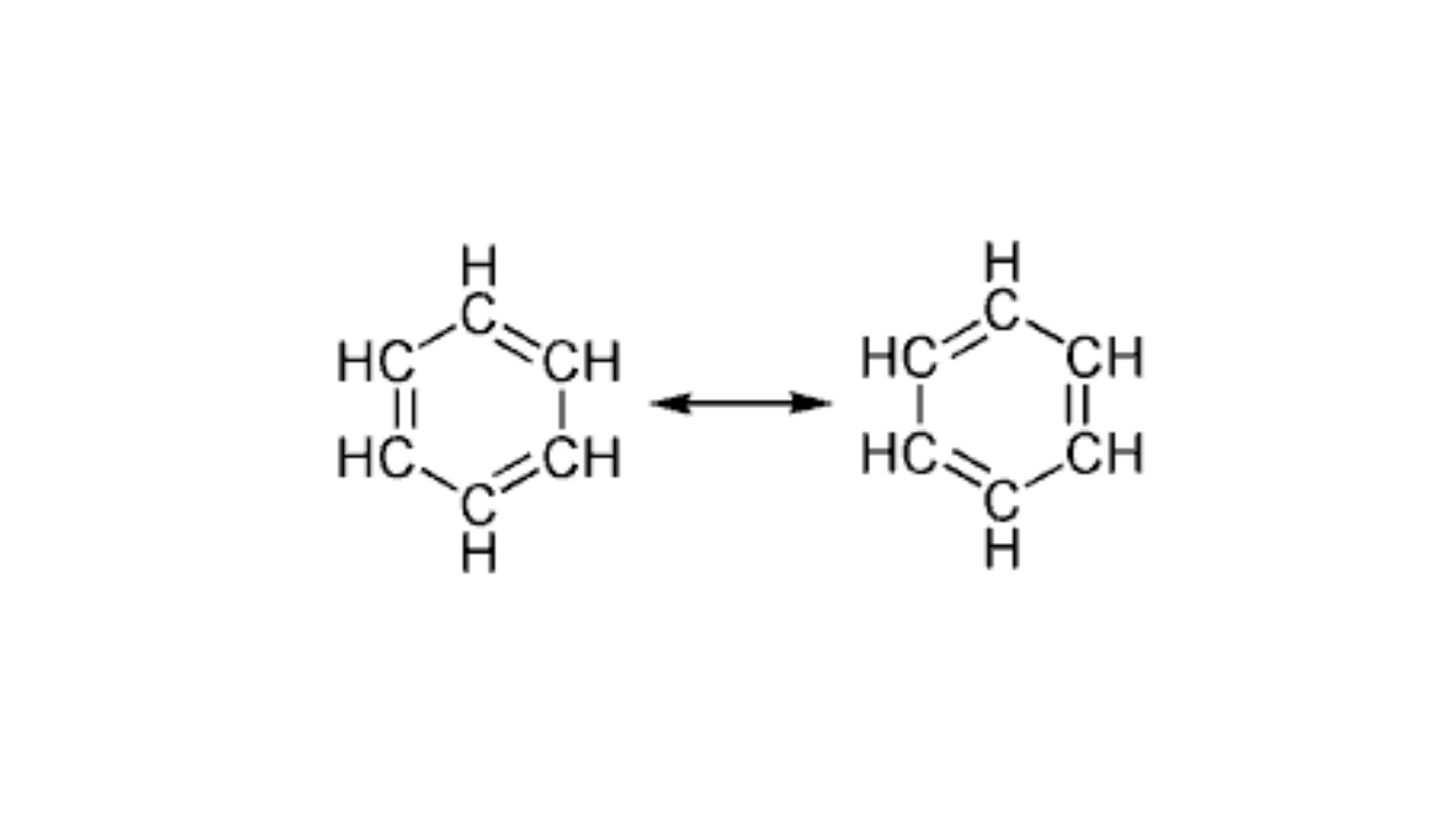
Benzene is composed by a simple aromatic ring in which the bond distances are all the same C-C. In addition to benzene, carboxylic acids are included among the delocalized electrons, which dissolved in water lead to the dissociation of the proton and the electrons, which are delocalized into two oxygen atoms.
Delocalized electrons are important for many reasons. The chemical reaction may not occur because the electrons delocalize in a stable configuration, for example by attempting a Friedel-Crafts alkylation of benzene with 1chloro-2methylpropane, the carbocation rearranges to a tert-butyl group stabilized by hyperconjugation, a particular type of delocalization.
Among the aromatic hydrocarbons, so called for their pungent and characteristic odour, benzene is the most unsaturated and has a very particular structural formula C6H6. The carbon atoms of the benzene chain are sp2 hybridized and unite with each other and with hydrogen thanks to \ sigmaσ bonds. The six remaining electrons overlap their p orbitals to form a single electronic cloud distributed over the entire molecule. The result is a very stable \ pi bond in which electrons are shared among all the carbon atoms, a situation that is called electronic delocalization.
Which hydrocarbons have electronic delocalization
The best known of the aromatic hydrocarbons is precisely benzene, which, as we have seen, passes from one chemical formula to another according to electronic delocalization.
In chemistry we speak of delocalized electrons and localized electrons, which are those that belong to a single atom and those that are inside a bond between two atoms. The former, on the other hand, are shared between several atoms.
The conditions that allow the electronic delocalization are presence of a P orbital that overlaps the P orbitals of the adjacent atoms; presence on the same plane of all atoms that share electrons. Aromatic compounds such as benzene, pyridine, and unsaturated alpha-beta systems such as acrolein fall under these conditions.
When we analyse a molecule with electron delocalization we cannot draw the electrons in a single bond because they are delocalized in the whole structure, so chemists write structures with localized electrons, which are resonance boundary structures that contribute to the real electronic structure of the molecule.
If you want to know more about the electronic delocalization of hydrocarbons, you can contact the specialists of Settala Gas, which has been producing, distributing and marketing hydrocarbons for various industrial uses since 1966. Contact us for more information!